Abstract
Introduction: CD25 is expressed in a proportion of non-Hodgkin lymphoma (NHL) cases, including subtypes such as peripheral T-cell (PTCL), cutaneous T-cell (CTCL), and diffuse large B-cell (DLBCL) lymphomas. ADCT-301 (camidanlumab tesirine [Cami-T]) is an antibody drug conjugate comprising a human monoclonal antibody against CD25 conjugated to a potent pyrrolobenzodiazepine dimer toxin. As CD25 is also expressed on regulatory T-cells, it is hypothesized that Cami-T may act as an immunomodulatory agent in addition to direct tumor cell targeting. Here, we report a first-in-human clinical trial of Cami-T, focusing on patients (pts) with relapsed/refractory (R/R) NHL.
Methods: This is a Phase 1, open-label, dose-escalation and dose-expansion multicenter study in pts with R/R NHL. The objectives of the study are to assess the safety and tolerability, determine the recommended dose(s) for expansion, and evaluate pharmacokinetics and pharmacodynamics of Cami-T. The study will also assess the clinical activity of Cami-T as measured by overall response rate (ORR; per 2014 Lugano Classification and Global Response Score Grading Scales for Modified Severity-Weighted Assessment Tool for CTCL), duration of response, progression-free survival, and overall survival. Pts receive Cami-T intravenously every 3 weeks (Q3W; 1 Cycle) intravenously. Dose escalation was performed according to a continual reassessment method.
Results: As of June 15, 2018, 39 pts with NHL have been enrolled in the study. Male (66.7%) and female (33.3%) pts had a median age of 67 years (range 33-88) at baseline and had received a median 3 prior therapies (range 1-12), with 9/39 (23%) pts having undergone prior stem cell transplant. Histological subtypes of NHL treated include DLBCL (n=14), mantle cell lymphoma (n=3), follicular lymphoma (n=1), Burkitt lymphoma (n=1), other B-cell lymphoma (n=4); CTCL (n=8), adult T-cell leukemia/lymphoma (ATLL; n=5), PTCL (n=3), and angioimmunoblastic T-cell lymphoma (AITL; n=1). Doses ranged from 3 to 150 µg/kg (median number of cycles: 2 [range 1-5], with a median treatment duration of 22 days [range 1-127]). Treatment-emergent adverse events (TEAEs) were reported in 38/39 (97.4%) pts; most common TEAEs (≥20%) for pts with NHL were diarrhea (11 [28.2%] pts), fatigue (11 [28.2%] pts), pyrexia (10 [25.6%] pts) and decreased platelet count (9 [23.1%] pts). Grade ≥3 TEAEs and TEAEs leading to treatment discontinuation occurred in 29 (74.4%) and 5 (12.8%) pts, respectively. There were immune-related AEs reported in 5 patients, comprising one case each of dermatitis exfoliative (Grade 3), thyroiditis (Grade 3), erythema multiforme (Grade 2), hypothyroidism (Grade 2), hyperthyroidism (Grade 2), and swelling face (Grade 1). The maximum tolerated dose has not been reached.
Exposure to conjugated antibody was dose-related, and following the 80 µg/kg dose was sustained throughout the dosage interval (mean average conjugated antibody concentration of 0.157 µg/mL [coefficient of variation {CV}=38.5%] and mean minimum concentration of 0.022 µg/mL [CV=99%]; n=9).
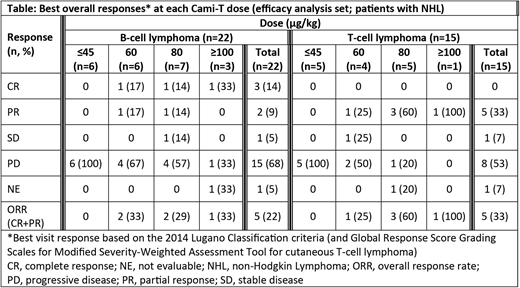
Response data for pts with NHL are shown in the Table. No responses were seen below 60 µg/kg. At doses 60-150 µg/kg, the ORR was 38.5% (10/26 pts) and the complete response (CR) rate was 11.5%. In pts with B-cell lymphoma, 16 have been treated with doses ≥60 µg/kg, with an ORR of 31.3% (5/16); CR was seen in 18.8% (3/16) pts and partial response (PR) in 12.5% [2/16] pts. Ten pts with T-cell lymphoma have been treated with doses ≥60 µg/kg, with an ORR of 50% (5/10; all PR); responses were seen in the following subtypes: 2 pts with CTCL (mycosis fungoides), 1 pt with ATLL, 1 pt with PTCL and 1 pt with AITL. Enrollment into 60 µg/kg and 80 µg/kg cohorts continues for pts with T-cell lymphomas to define the optimal dose for expansion.
Conclusions: In pts with R/R NHL, active doses of Cami-T with acceptable safety profiles were identified for both B-cell and T-cell lymphoma during dose escalation of this study. Five out of the 10 pts on study with T-cell lymphoma treated in the 60, 80, or 100 µg/kg cohorts responded. Subtype-specific cohorts in the dose escalation portion of this study are underway to better define the recommended dose for expansion.
Study sponsored by ADC Therapeutics. http://clinicaltrials.gov/show/NCT02432235.
Collins:MSD: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Celleron: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; Amgen: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Pfizer: Consultancy, Honoraria; Celgene Corporation: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Celgene Corporation: Research Funding. Horwitz:Seattle Genetics: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Aileron Therapeutics: Consultancy, Research Funding; Innate Pharma: Consultancy; Forty Seven: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Corvus: Consultancy; Portola: Consultancy; Mundipharma: Consultancy; Kyowa-Hakka-Kirin: Consultancy, Research Funding; Spectrum: Research Funding; Trillium: Consultancy; Infinity/Verastem: Consultancy, Research Funding. Davies:Gilead: Honoraria, Research Funding; Karyopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Research Funding; F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite: Consultancy; Acerta Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Karnad:ADC Therapeutics: Research Funding. Samaniego:ADC Therapeutics: Research Funding. Spira:BMS: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; ADC Therapeutics: Research Funding; Roche: Consultancy. Fields:Takeda: Speakers Bureau; Roche: Speakers Bureau; MSD: Speakers Bureau; ADC Therapeutics: Research Funding. Menne:Gilead: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; ADC Therapeutics: Research Funding. Boni:ADC Therapeutics: Employment, Equity Ownership. Cruz:ADC Therapeutics: Employment, Equity Ownership. Feingold:ADC Therapeutics: Employment, Equity Ownership. He:ADC Therapeutics: Employment, Equity Ownership. Wuerthner:ADC Therapeutics: Employment, Equity Ownership. Hamadani:Cellerant: Consultancy; Celgene Corporation: Consultancy; Ostuka: Research Funding; Takeda: Research Funding; MedImmune: Consultancy, Research Funding; Merck: Research Funding; Janssen: Consultancy; ADC Therapeutics: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal